All-copper
-
Making a thread for a potential all-copper chemistry, which came up in discussion during our regular meeting today as a potential safe chemistry for testing, particularly as we scale to larger electrolyte volumes/cell areas. H/t to @danielfp@chemisting.com !
The voltage is too low to be of major commercial interest (~ 0.6 V), but in the charged state it's not volatile like iodine-containing complexes.
It also fulfills our emerging criteria:
- Safe (in comparison to vanadium or lead-based aqueous systems)
- Accessible (low-cost and available to amateur chemists)
- Compatible with porous separators (no requirement for ion-exchange membrane)
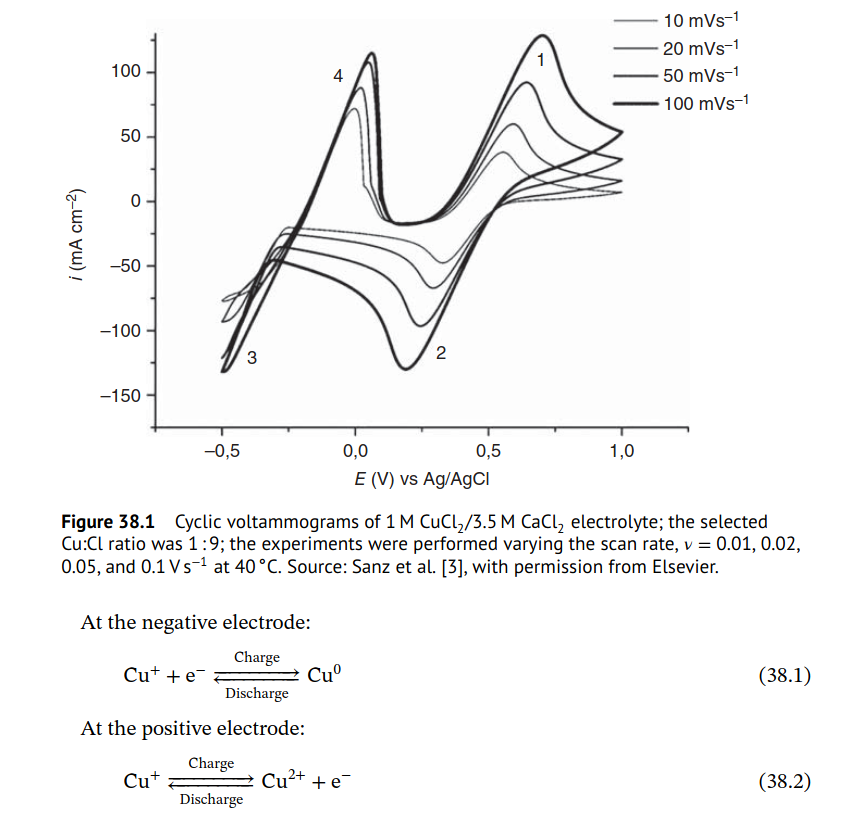
From Roth et al. chapter 38 on all-copper [1]:
The CuFB is a novel aqueous system based on the three oxidation states of copper, achieved by stabilizing the Cu(I) complexes (CuCl2−, CuCl32−) in concentrated chloride solutions. The resulting cell has a hybrid configuration with the chemical reactions shown in Eqs. (38.1) and (38.2).
And the self-discharge reaction for completeness:
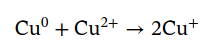
We are considering testing this in the dev kit and then in our first large-format cells, as it could be cheaper and safer on the 100-1,000 mL scale than zinc-iodide.
[1] Roth, C. et al. (eds.) (2023). Flow Batteries: From Fundamentals to Applications, Wiley.
-
 K kirk referenced this topic on
K kirk referenced this topic on
-
 K kirk referenced this topic on
K kirk referenced this topic on
-
I just now read about this chemistry. Did you do any testing of it yet? Why is the voltage too low for commercial isnterest? Because you'd need such big stacks (with high shunt losses) to get any decent voltage?
@sepi This chemistry has 2 large problems.
-
The Cu+ is VERY oxygen sensitive, so to run it successfully at any scale, significant inert gas purging of the system is essential. The systems also need to be quite air tight as any leaks that lead to air porosity will kill it, unless you're in a glove box or another form of inert environment.
-
It has VERY slow kinetics. This means the systems need to be run at around 40-50C in order for the efficiency to be good. The values you commonly see in the literature are for systems that are run at 40-50C.
While the chemistry seems simple, which is the reason why we have considered it, upon studying it more carefully it seems that these problems would make it too unfriendly for our kit and our larger scale cell. Large scale purging of solution and keeping large volumes of solution at 40-50C are both things we would want to avoid doing.
-
-
I didn't know iodine was explosive. Did a google and yea, when mixed with other stuff like zinc oxide and water :(. So, googled ion exchange membrane for sale and was seeing what looked like rolls of it at amazon. But those rolls were small, some ~50cm square and still pushing $100.
The googled diy. There are a lot of YT vids. Think I'll be watching some of those tonight.
-
@sepi This chemistry has 2 large problems.
-
The Cu+ is VERY oxygen sensitive, so to run it successfully at any scale, significant inert gas purging of the system is essential. The systems also need to be quite air tight as any leaks that lead to air porosity will kill it, unless you're in a glove box or another form of inert environment.
-
It has VERY slow kinetics. This means the systems need to be run at around 40-50C in order for the efficiency to be good. The values you commonly see in the literature are for systems that are run at 40-50C.
While the chemistry seems simple, which is the reason why we have considered it, upon studying it more carefully it seems that these problems would make it too unfriendly for our kit and our larger scale cell. Large scale purging of solution and keeping large volumes of solution at 40-50C are both things we would want to avoid doing.
@danielfp248 ahh, I had't caught those two caveats. Especially the oxygen sensitivity seems to be a big issue to me. That makes it very unfriendly to tinkering. The temperature requirement is off course also problematic, probably also from an efficiency point of view.
On top of that it's also a hybrid, so I guess has all the problems with dendrites etc.
-
-
@danielfp248 Any reason making a membrane was abandon? Seemed like you were making progress and without it you seem more limited on chemistry.
-
@danielfp248 Any reason making a membrane was abandon? Seemed like you were making progress and without it you seem more limited on chemistry.
@Vorg There are 3 reasons.
First is that membranes have to be extremely robust to be viable as even minimal migration will cause the electrolytes to mix with time, which prevents any large scale application.
Second is that selective membranes are completely damaged by dendrites while microporous membranes survive them without problems. Use of a membrane completely eliminates the possibility of using Zn anodes in practice. This eliminates some great chemistries for large scale applications (like Zn/I).
Third is that making these DIY membranes in a reproducible way is hard, I had a lot of problems reproducing my results and the inconsistencies in both chemical and physical properties made this hard to pursue. This would only be worse for larger area membranes.
I think it is a very interesting field to pursue if you're interested but I would like to focus my energy elsewhere - mainly the larger scale kit - due to the above issues.
-
Do I understand correctly that microporous membranes mostly let pass small ions and "try" to contain the active speices like I and Zn? In reality though they might let them pass in non-negligable qunatities, no? Is this what you call migration?
@sepi Yes. Microporous membranes let everything pass in proportion to their diffusion coefficient (which is related with their charge and size) and their concentration gradient. The good thing for Zn/I is that the charged species on the anode side is a solid (so it doesn't diffuse through the membrane) and the I3- is a larger charged species, so it diffuses more slowly than I- and K+. When using Triethylene glycol the Trieg_I5- complex is also much larger, so diffuses even more slowly across (this is why CE values when using Trieg tend to be higher too). They DO let a lot of charged catholyte migrate through the membrane (especially at high SOC values) and react with the solid Zn, so this is why the CE values are never really close to 100% (the battery always self-discharges to some extent due to migration). This migration of course requires circulation, in practice if you charge the battery and stop pumping the charge state is held until you want to discharge.
In the case of Zn/I a big positive is that charged catholyte species that diffuse through the membrane also react with dendrites, so the dendrites are kept from shorting the battery. If they get to shorting the battery then doing lower current charge/discharge cycling can also eliminate them. In this case, microporous membranes are what guarantees the long term reliability of the system.
-
@Vorg There are 3 reasons.
First is that membranes have to be extremely robust to be viable as even minimal migration will cause the electrolytes to mix with time, which prevents any large scale application.
Second is that selective membranes are completely damaged by dendrites while microporous membranes survive them without problems. Use of a membrane completely eliminates the possibility of using Zn anodes in practice. This eliminates some great chemistries for large scale applications (like Zn/I).
Third is that making these DIY membranes in a reproducible way is hard, I had a lot of problems reproducing my results and the inconsistencies in both chemical and physical properties made this hard to pursue. This would only be worse for larger area membranes.
I think it is a very interesting field to pursue if you're interested but I would like to focus my energy elsewhere - mainly the larger scale kit - due to the above issues.
@danielfp248 said in All-copper:
First is that membranes have to be extremely robust to be viable as even minimal migration will cause the electrolytes to mix with time, which prevents any large scale application.
Do you refer here to an actual puncture in the membrane or to the incomplete selectivity like with porous membranes? The porous membranes also seem to allow minimal migration, no? That would mean they would also preclude large scale applications?
-
@danielfp248 said in All-copper:
First is that membranes have to be extremely robust to be viable as even minimal migration will cause the electrolytes to mix with time, which prevents any large scale application.
Do you refer here to an actual puncture in the membrane or to the incomplete selectivity like with porous membranes? The porous membranes also seem to allow minimal migration, no? That would mean they would also preclude large scale applications?
@sepi I am talking about incomplete selectivity. Ion selective membranes are necessary when your anolyte and catholyte are different, however any significant migration due to imperfect selectivity will result on intermixing, which eventually leads to the electrolytes becoming useless. If large scale systems have to operate in a scale of decades, this is not acceptable, even if the selectivity is very high. Puncturing of these membranes strongly accelerates the process, but even under ideal conditions migration will slowly get you there. This is why great asymmetric chemistries - such as all-soluble Fe - are yet to reach commercial applications. Not only are the cost of the membranes a factor, but your electrolyte gets ruined as a function of time.
For large scale applications, even when using selective membranes, symmetric electrolytes - equal catholyte and anolyte composition on discharged state - are needed so that this problem can be avoided (reason why Vanadium works so well). However any symmetric system can be used with a microporous membrane at lower CE. Since a symmetric system has equal composition on discharged, the tanks can be routinely intermixed when fully discharged to make sure no imbalances remain in the system.
-
I know we kinda got off topic, so bringing it back. When Kirk in the Op said iodine is volatile, i googled I iodine explosive and got this:
"Iodine forms explosive or shock-sensitive compounds when mixed with REDUCING AGENTS (such as LITHIUM, SODIUM, ALUMINUM and their HYDRIDES) and liquid AMMONIA. Iodine will ignite POWDERED METALS (such as ANTIMONY, MAGNESIUM and ZINC) in the presence of WATER."
I read this to say that if that membrane ruptured, the two sides would mix and BOOM. I don't know much about photo paper as opposed to regular paper. I'm thinking it's paper with some kind of polymer coating. When paper gets wet, it breaks down. So prolonged setting between two fluids, seems like the boom is just a matter of time.
So with this copper, If you get air in it, say need to work on it, is it all ruined? or do you just put a vacuum on it and repressurize with something like Argon and you're good to go again? As for the temperature it needs, I live in tucson, We hit 113 a couple weeks ago with normal daily temps 100+ in the summer, so only a problem in the winter.

-
I know we kinda got off topic, so bringing it back. When Kirk in the Op said iodine is volatile, i googled I iodine explosive and got this:
"Iodine forms explosive or shock-sensitive compounds when mixed with REDUCING AGENTS (such as LITHIUM, SODIUM, ALUMINUM and their HYDRIDES) and liquid AMMONIA. Iodine will ignite POWDERED METALS (such as ANTIMONY, MAGNESIUM and ZINC) in the presence of WATER."
I read this to say that if that membrane ruptured, the two sides would mix and BOOM. I don't know much about photo paper as opposed to regular paper. I'm thinking it's paper with some kind of polymer coating. When paper gets wet, it breaks down. So prolonged setting between two fluids, seems like the boom is just a matter of time.
So with this copper, If you get air in it, say need to work on it, is it all ruined? or do you just put a vacuum on it and repressurize with something like Argon and you're good to go again? As for the temperature it needs, I live in tucson, We hit 113 a couple weeks ago with normal daily temps 100+ in the summer, so only a problem in the winter.

@Vorg The reaction is not of elemental iodine with Zinc metal, it is of triiodide with Zinc metal. While this reaction is very exothermic, it is not explosive in this context because of a few reasons:
-
While all the Zn is deposited at the same spot the oxidized iodine is distributed through the entire catholyte volume, so if the membrane ruptures it takes a while for the reaction to happen, it doesn't all happen at once.
-
There is a lot of water here, which carries a lot of thermal load. While you can generate a lot of heat on membrane rupture, it isn't even enough to boil the solution at 100% SOC (from my experience). Per point 1, only a small fraction of iodine is able to react at any given point and for more to react flow must be present.
-
Zn here is bulk Zn, it is not powedered or finely divided at all
I have seen this happen experimentally at high SOC at 2M KI using photopaper. The membrane ruptured at high SOC and what I saw was the volume all shift mostly to one side and the potential drop very quick. Nothing exploded, melted or anything that dangerous. Untreated photopaper is obviously not intended to be a long term use membrane, it is intended as a viable membrane to carry out short term testing that is very easily accessible. To use it long term it is necessary to increase its lifetime with a PVA coating or something along these lines.
The most dangerous scenario in my experience is solid iodine forming in the cathode, blocking flow and causing tubing to unhook, this then splashes charged electrolyte, which is a considerably greater hazard. The use of triethylene glycol seeks to mostly prevent this scenario, but it is still possible if very high currents are used or the cell is overcharged to high potentials (>1.6V).
With the above said, the anolyte and catholyte hold a lot of energy and mixing them obviously instantaneously discharges all that energy. At 10mL of total volume this is not much, around 250mWh but when using larger volumes this is a considerable risk and larger scale devices must be created and tested with this potential scenario in mind.
About the copper, oxygen in it would ruin the electrolyte because it would oxidize Cu+ to Cu+2 and this reaction would also increase the pH of the anolyte. This would eventually cause Cu hydroxide to fall out and kill the device. To recover the cell you would need to purge the electrolyte with Argon, cycle the solution over Cu metal to reduce the oxidized Cu2+ back to Cu+ and add hydrochloric acid to adjust the pH back to the proper level if necessary. To run a cell like this you need to make the system quite airtight and ensure the electrolyte is purged with Argon from the start.
An idea to test for a period under oxygen-present conditions is to keep a piece of sacrificial copper in the anolyte reservoir to make sure that any Cu2+ that is formed is reduced back, so that way you would only succumb to the slow pH up creep. However this means that the CE you measure isn't really fair, because you are not accounting for a huge chunk of active material present there. It can be useful however to cycle and perhaps get some insights into other sections of the system under a regular atmosphere. However I don't honestly know if this is good enough, as the reaction of Cu+ with oxygen is quite fast.
The copper chloride (I) initial electrolyte is usually prepared like this (under copper metal) to make sure there is no Cu2+ present when first loading the electrolyte into the device.
-
