Sourcing parts
-
About the triethylene glycol: can it be substituted for some other glycol maybe? I find it slightly hard to find. What's its purpose anyways?
@sepi No, it absolutely has to be triethylene glycol. Other glycols won't work, its chemical structure is exactly required to stabilize I5- in solution. If you're in the EU, you can get it from laboratodiumdiscounter in the Neatherlands (https://www.laboratoriumdiscounter.nl/es/trietilenglicol-99-extra-puro.html). It is not very expensive, 1L is like 15 EUR.
It's main purpose is to stabilize higher order polyiodides in solution and therefore prevent solid I2 formation at high SOC values. However if you don't go to high SOC values (keep the battery charge state low, then you can do without it).
-
Ahh, excellent. Thanks for both the new reagents source and the explanation. ChatGPT told me that Propylene glycol might work, but then again why trust it :).
@sepi It doesn't, propylene glycol was also tested on the research paper that found triethyleneglycol worked. You need a very specific structure to stabilize the polyiodides.
-
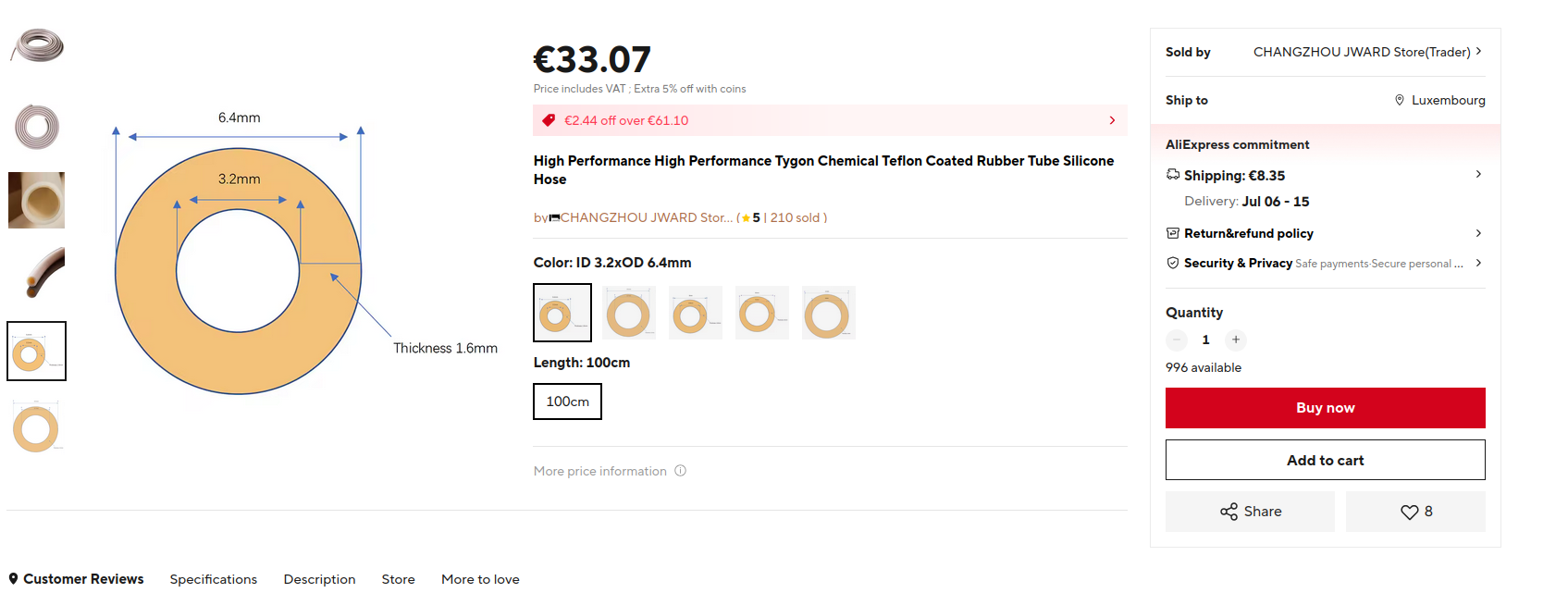
@danielfp248 i just received the tubing and I think I ordered the wrong one. I guess I didnct read correctly what you wrote to me about it. I got PharMed BPT tubing that is not lined with teflon/ptfe. Am I correct that I should order the teflon lined one explicitely? Also how did you know that the one I originally wanted to order (as seen on screenshot) is not good?
-
@danielfp248 i just received the tubing and I think I ordered the wrong one. I guess I didnct read correctly what you wrote to me about it. I got PharMed BPT tubing that is not lined with teflon/ptfe. Am I correct that I should order the teflon lined one explicitely? Also how did you know that the one I originally wanted to order (as seen on screenshot) is not good?
@sepi The pharMed BPT is not the correct tubing. It will work for a while but will slowly leak iodine at high SOC values (especially above 50% SOC). You should order the teflon lined one explicitly, they refer to it as "tygon chemical tubing". Make sure to confirm they are sending you PTFE lines tubing. As I mentioned they do NOT sell this through aliexpress so you will need to contact them directly.
Note that you can still run tests at lower SOC values with normal BPT tubing.
-
@danielfp248 did I understand correctly that Ti foil can be used to replace the grafoil bipolar plate/current collector? You talk about Ti foil as electrode. I'm not so sure about nomenclature here anymore.
-
@danielfp248 did I understand correctly that Ti foil can be used to replace the grafoil bipolar plate/current collector? You talk about Ti foil as electrode. I'm not so sure about nomenclature here anymore.
@sepi The Ti foil can be used to replace the grafoil electrode. Ti can also be used to replace the current collector entirely, but then it would have to be a Ti plate and machining thick Ti is harder plus the conductivity of Ti is much lower than copper. You can use a brass/copper current collector and then have a thin Ti foil used for the electrode material instead of grafoil.
-
Ok, great, that's what I'm aiming for atm. Thanks for the quick reply. Btw I created 2 PRs on codeberg about docs. Maybe have a look if you find the time.
-
Ok, great, that's what I'm aiming for atm. Thanks for the quick reply. Btw I created 2 PRs on codeberg about docs. Maybe have a look if you find the time.