Fe-Mn
-
@danielfp248 I ran into some of your old work on Fe-Mn batteries. I've been interested in Fe-Mn batteries for some time and was wondering if you could share some of your experience.
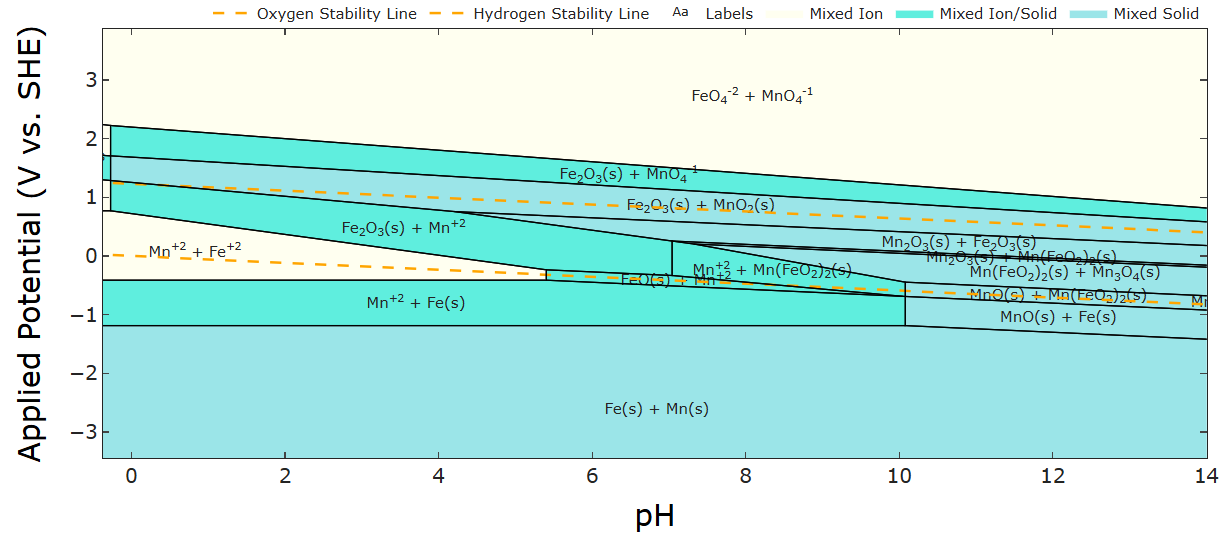
In particular, if we look at the pourbaix diagram of Fe and Mn overlapped, there's a region in the pH 4-6 range where Mn2+ oxidizes to MnO2 and Fe2+ reduces to Fe on charge.
Let's say we can ignore the fact that there are solid phases - then ion crossover poses a long term concern. Have you guys found any inexpensive or DIY ion selective membrane options (either specific to a particular ion or broad spectrum diy anion/cation exchange membranes?) In my experience screening for inexpensive battery chemistries, crossover of solution phase species is a problem I haven't really seen an easy DIY solution for. Not that I've looked super hard!
-
@danielfp248 I ran into some of your old work on Fe-Mn batteries. I've been interested in Fe-Mn batteries for some time and was wondering if you could share some of your experience.
In particular, if we look at the pourbaix diagram of Fe and Mn overlapped, there's a region in the pH 4-6 range where Mn2+ oxidizes to MnO2 and Fe2+ reduces to Fe on charge.

Let's say we can ignore the fact that there are solid phases - then ion crossover poses a long term concern. Have you guys found any inexpensive or DIY ion selective membrane options (either specific to a particular ion or broad spectrum diy anion/cation exchange membranes?) In my experience screening for inexpensive battery chemistries, crossover of solution phase species is a problem I haven't really seen an easy DIY solution for. Not that I've looked super hard!
@muntasirms I've done several experiments on Fe/Mn and Zn/Mn chemistries. The problem with Mn2+ is the formation of the solid MnO2 phase and the presence of the metastable Mn3+. Forming solid MnO2 poses a non-trivial constraint on the battery, as it limits deposition per area to around a few mAh/cm2, very impractical for a flow battery, furthermore, Mn3+ formation causes MnO2 to form away from the electrode (as it disproportionates into Mn2+ and MnO2), causing some Mn to become lost around the battery system.
A possibility is to try to stabilize Mn3+ somehow (for example with Mn-EDTA), but the main issue is that even this stabilized Mn3+ is unstable and eventually self-degrades by oxidizing the chelate around the Mn atom. I tried creating a flow battery system with Fe-DTPA/Mn-EDTA, which has a max solubility of around 0.5M, but the system did not cycle due to the Mn-EDTA being too unstable. There are a few posts on my blog about this. The oxidized Mn-EDTA is also quite sensitive to pH, so it is hard to create conditions under which it is stable. Mn3+ can also be stabilized with HCl+H2SO4, but only at very low concentrations (there's a paper on using this in a flow battery, but only very low capacities are achieved).
Another possibility is to stabilize MnO2 as nanoparticles in solution. This can be achieved through the use of TiO2+ in sulfuric acid (using titanyl sulfate). Such systems are quite harsh from a chemical perspective, so I haven't tested them at all (I don't want to run a 3M sulfuric acid system containing reactive Ti compounds). You can read more about this system here (https://www.sciencedirect.com/science/article/pii/S0378775322000209). This is one of the most interesting and potentially viable Mn chemistries out there although only reaching around 17Wh/L.
Honestly Mn based systems are best suited for static batteries, where the formation of the MnO2 and Mn3+ phases is less problematic.
-
I was doing a literature review of Fe/Mn the other day and happened to find this article on Fe/Mn using MSA (https://www.sciencedirect.com/science/article/pii/S001346862030637X). This article uses an asymmetric setup with FeCl3 on one side (paper says it's FeCl2 but that must be a mistake because the reaction requires Fe reduction on charge) and MnCl2 on the other, both sides using 3M methanesulfonic acid, separated by a Nafion membrane. The Mn3+ is in theory stabilized in the acid media, but given the color of the solution it might be that MnO2 nanoparticles are stabilized instead.
While the paper does not use this in a symmetric setup, I see no reason why this reaction couldn't work symmetrically so I prepared an electrolyte using the following:
- 3g MnCl2.4H2O
- 4mL FeCl3 40% w/w solution
- 4mL 75% methanesulfonic acid (MSA)
- around 1mL of water (final volume was taken to 10mL)
The above creates a solution that is around 1.5M Fe, 1.5M Mn and 3M MSA. This setup has the advantage that both reactions generate no solid products. At a 100% SOC this would give us ~20Ah/L. On charge:
Fe3+ + e- -> Fe2+
Mn2+ -> Mn3+ + e-The potential difference between these two half reactions is not very high though, so the total expected cell voltage is ~550mV. However this is a "true flow battery" in that power and capacity are fully decoupled as the reaction products are all in solution. Note that Mn3+ is expected to have limited stability, especially at high concentrations, so I would expect capacity to degrade heavily as the Mn3+ gets converted into MnO2, unless this MnO2 is somehow stabilized in solution (which could be as nanoparticles). Interestingly Fe2+ can react with MnO2, so the battery might also self-heal if this happens, just temporarily capacity in the process.
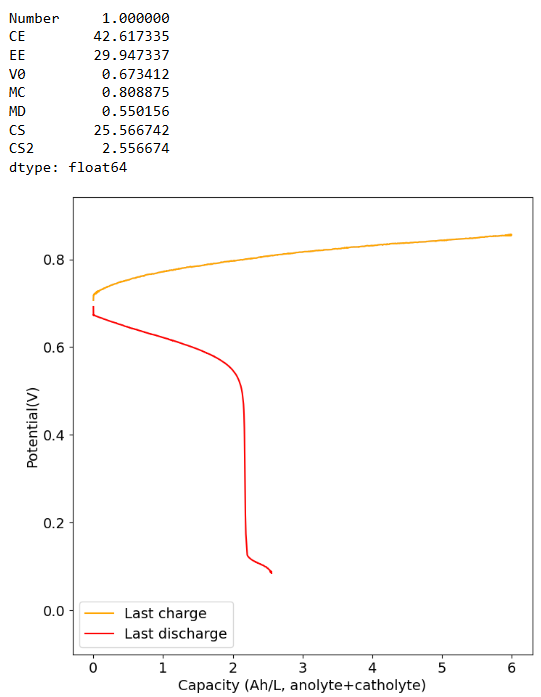
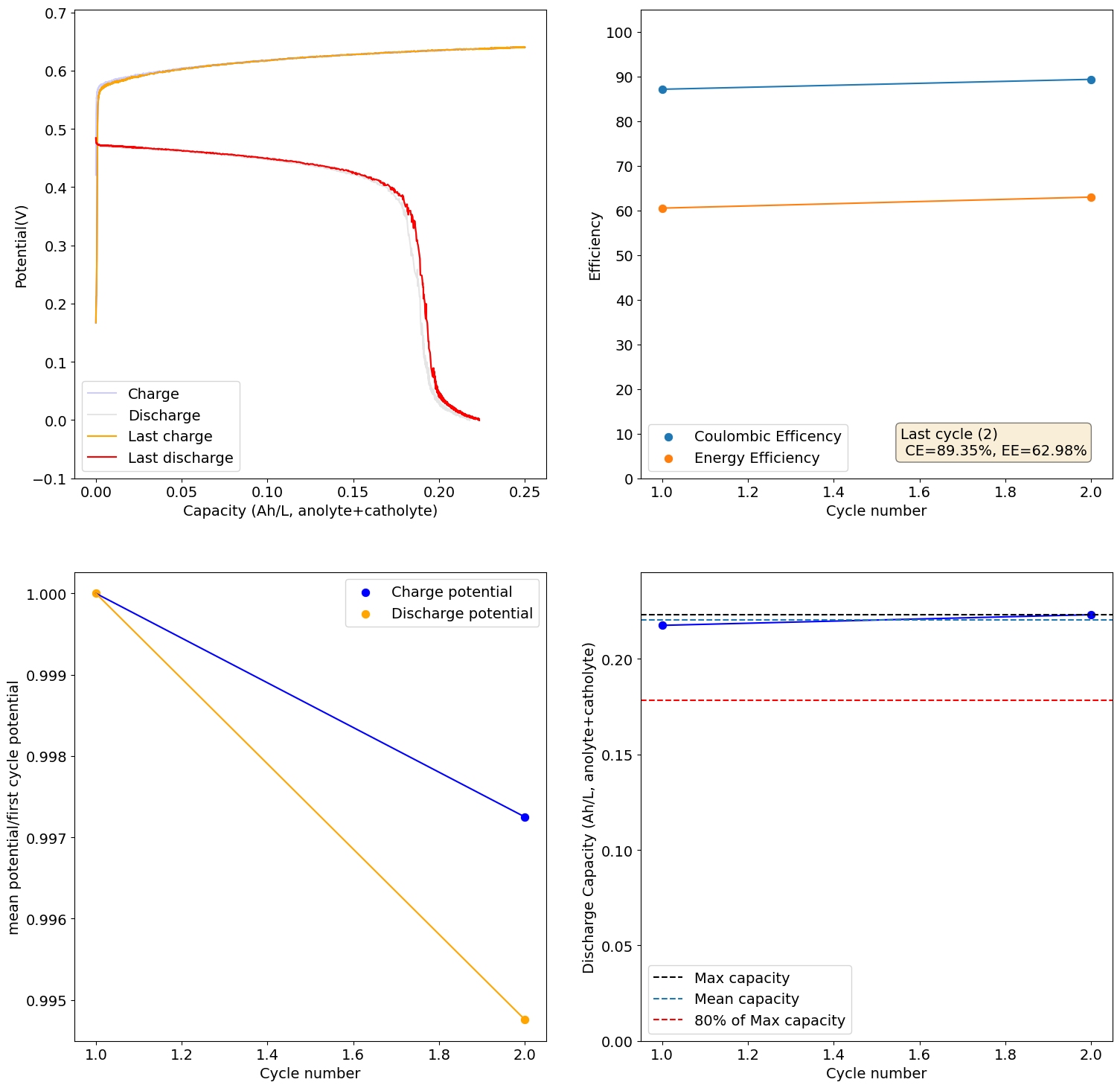
I loaded the electrolyte in a cell with carbon felt on both anode and cathode and used Daramic as a separator (cannot use paper as it reacts with Mn3+). Below are the results of a few cycles at low capacity (0.25Ah/L at 10mA/cm2), just to test the chemistry. It seems to work quite well:
I will continue to run some tests and will let you know what I get.
-
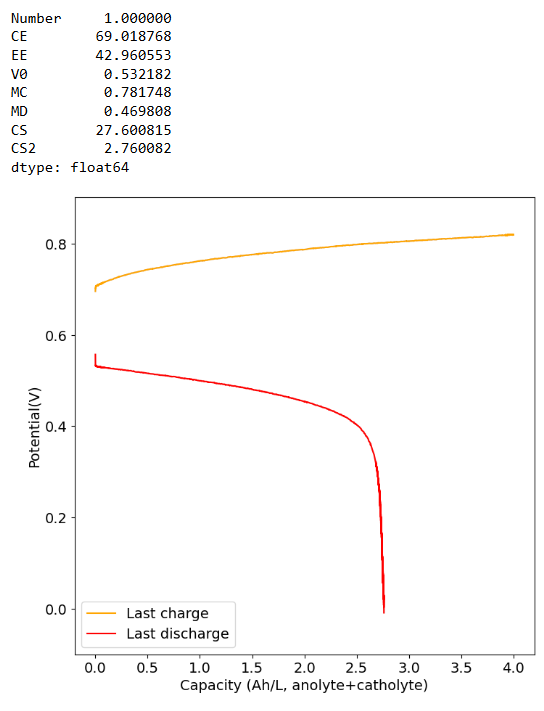
The CE drops a lot when going to higher capacities (even at a current of 40mA/cm2, which means it is not due to crossover as lower currents do not imply a lower CE). This is likely because the stability of Mn3+ species in solution is very limited, so you seemingly cannot exceed a ~2.5Ah/L capacity.
-
Charging to 6Ah/L at 30mA/cm2 and discharging at 5mA/cm2. At most we only get 2-3 Ah/L of available capacity, same as if we charged to 4Ah/L.