Alternative Electrolytes
-
I am now testing 7g ZnCl2, 0.84g FeCl2.2H2O and 0.8g Glycine plus 7mL of water, which gives around 10mL total volume (measured with a syringe). This is around 6M Zn, 0.5M Fe, 1M Glycine. This is how the electrolyte looks on preparation:

I am now testing it with a non-conductive felt on the Zn side, to increase the time it takes for dendrites to form, as they now have to cross the entire cell to reach the membrane. I expect some loss in conductivity but this can be worth the tradeoff. This also increases the time it takes for Fe3+ to react with Zn as it now has to entirely cross the separator and cannot easily meet Zn half way through.
As you can see the solution is not entirely translucid, because there is some slight Fe oxide impurity in the FeCl2 I use.
-
Charging to 2Ah/L (~30% of SOC at 0.5M Fe) shows promising results, although CE and EE are still quite low. I will try charging to 5Ah/L next.
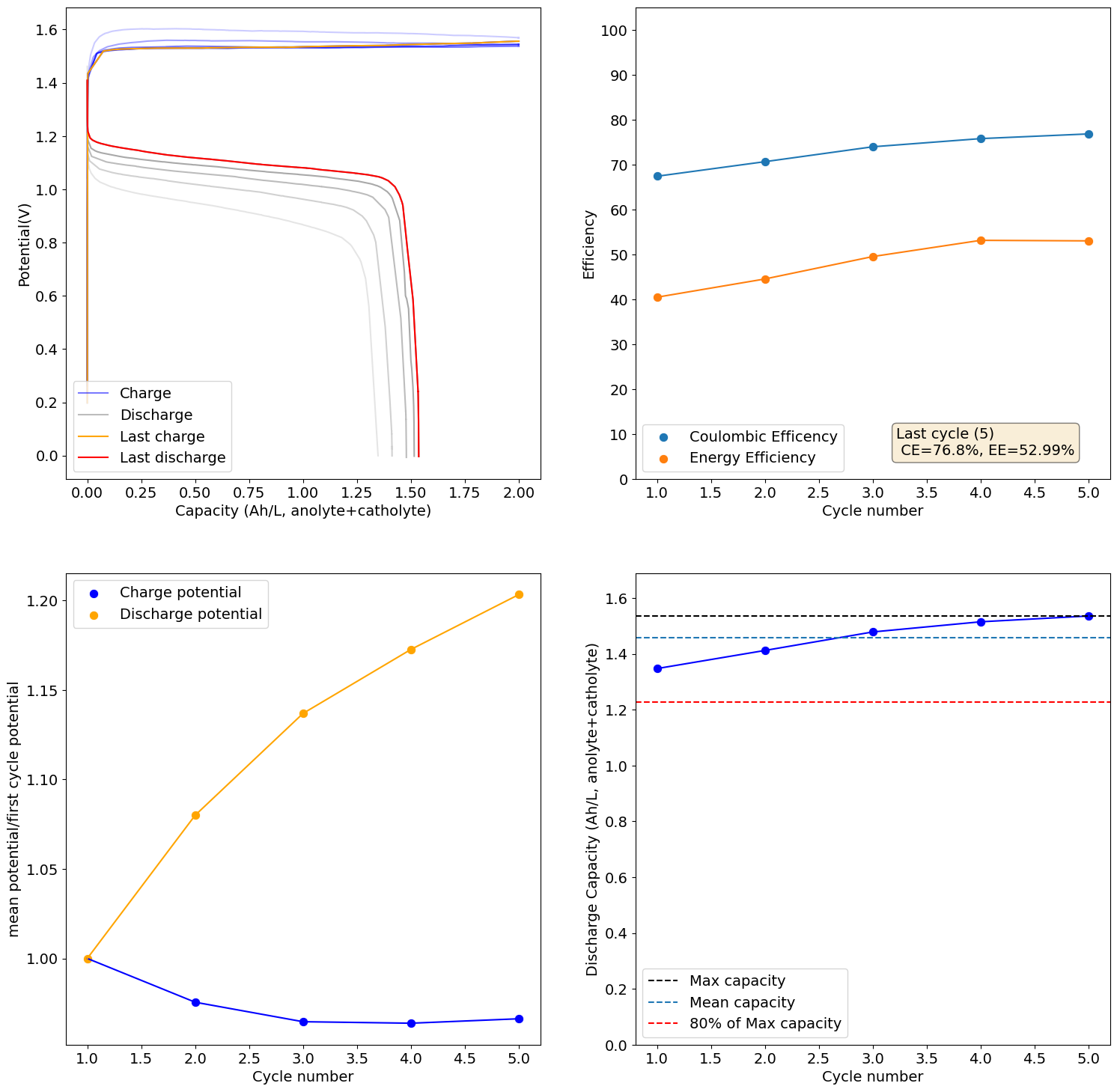
-
I also prepared a test electrolyte with 6.8g of ZnCl2, 3.2g of FeCl2.2H2O, 2.25g of Glycine with 5.5mL of water to reach a volume of 10mL and concentrations of 5M Zn, 2M Fe and 3M Glycine. The electrolyte is deep red as shown below. The 100% SOC mark for this electrolyte would be ~26Ah/L but I would honestly be more than happy if it cycled to 15Ah/L in a stable manner. I will test this electrolyte once I'm done testing the 0.5M Fe electrolyte I'm running atm.

-
The 0.5M Fe solution at a current density of 5mA/cm2 reached the Nernst limit at around 3.1Ah/L. This is the first cycle:
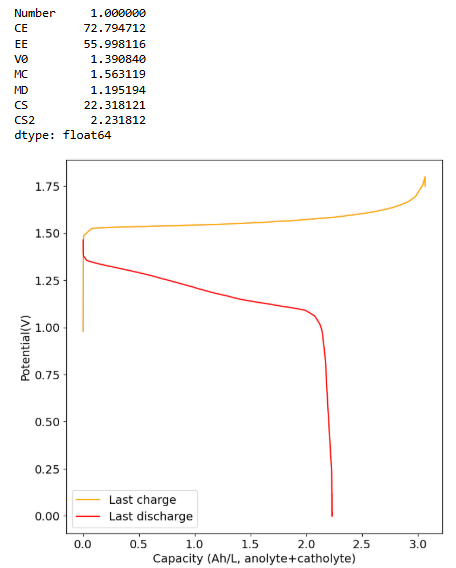
I will keep you posted on how the cycling goes and if it degrades in capacity as other similar tests have done in the past.
-
Same result as previous times, significant decreases in capacity as a function of cycling:
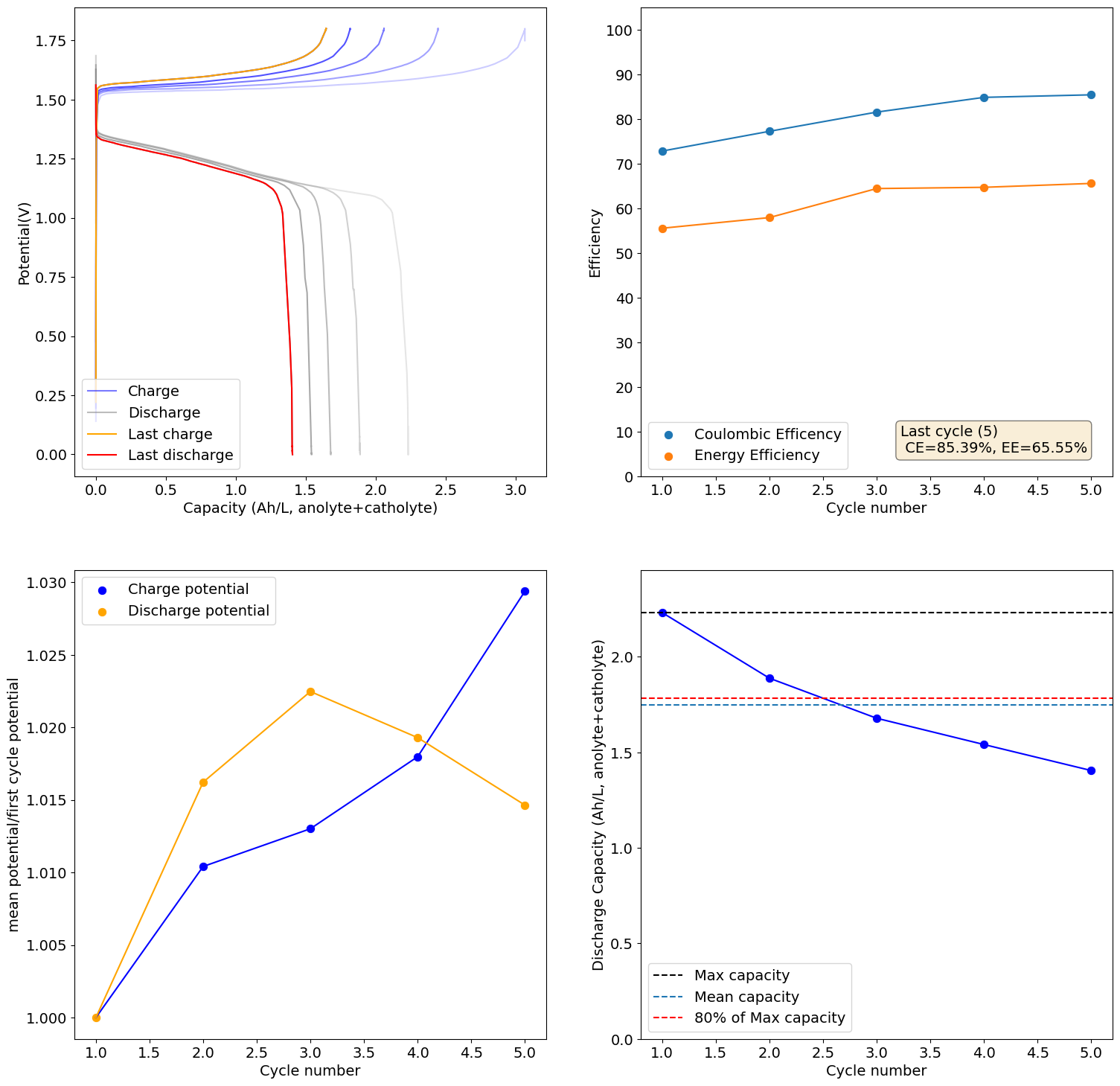
-
Question, what about using some sort aluminum oxide? Tesla is rumored to be using a new aluminum battery the developed in place of LFOP for the new model 2. They said something about aluminum holding 3 ions? per molecule instead of 1. I poked around and there are groups working on aluminum flow batteries. Seem they don't have the dendrite problem.
I see a number of groups working on aluminum flow batteries. Seems they don't have the dendrite problem.
-
Question, what about using some sort aluminum oxide? Tesla is rumored to be using a new aluminum battery the developed in place of LFOP for the new model 2. They said something about aluminum holding 3 ions? per molecule instead of 1. I poked around and there are groups working on aluminum flow batteries. Seem they don't have the dendrite problem.
I see a number of groups working on aluminum flow batteries. Seems they don't have the dendrite problem.
@Vorg Aluminum would be great in that it is a trivalent cation, so you get 3 electrons per Al atom, one of the most efficient atoms in this manner. A rechargeable Al/air battery has one of the highest theoretical energy densities possible, especially if the oxygen would come from the air. However aluminum is incredibly hard to reduce, so it is one of the trickiest batteries to get into a rechargeable form. In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems. I don't believe this is something we could realistically achieve, given the complexities of this chemistry. In my opinion there are lower hanging fruit, but of course, anyone who wants to try it and share is welcome!
-
Maybe that is what the video was talking about when they said the biggest problem is ion lock, or maybe it was electron lock. But basicly it's very hard to get the charge out at a useful rate. I'm sure there is more to Tesla's secret sauce, but they said Tesla's fix was very thin aluminum with carbon pressed into it creating a battery that gave it's charge better, could be charged in 5 minutes, and produced far less heat then lithium batteries with no thermal runaway problem. It sounded like they won't even use battery cooling for it because what heat it does produce makes it work better. A lot of "sounds good", but will see when it hits the road.
-
@Vorg Aluminum would be great in that it is a trivalent cation, so you get 3 electrons per Al atom, one of the most efficient atoms in this manner. A rechargeable Al/air battery has one of the highest theoretical energy densities possible, especially if the oxygen would come from the air. However aluminum is incredibly hard to reduce, so it is one of the trickiest batteries to get into a rechargeable form. In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems. I don't believe this is something we could realistically achieve, given the complexities of this chemistry. In my opinion there are lower hanging fruit, but of course, anyone who wants to try it and share is welcome!
@danielfp248 said in Alternative Electrolytes:
In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems.
What's expensive about the non-aqueous media? Do you speak about deep eutectic solvents?
-
Maybe that is what the video was talking about when they said the biggest problem is ion lock, or maybe it was electron lock. But basicly it's very hard to get the charge out at a useful rate. I'm sure there is more to Tesla's secret sauce, but they said Tesla's fix was very thin aluminum with carbon pressed into it creating a battery that gave it's charge better, could be charged in 5 minutes, and produced far less heat then lithium batteries with no thermal runaway problem. It sounded like they won't even use battery cooling for it because what heat it does produce makes it work better. A lot of "sounds good", but will see when it hits the road.
@Vorg This is a different type of battery technology though, it is aluminum Ion, using thin film materials. This type of battery chemistry isn't really compatible with a flow battery mechanic.
-
@danielfp248 said in Alternative Electrolytes:
In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems.
What's expensive about the non-aqueous media? Do you speak about deep eutectic solvents?
@sepi Just that water is very cheap, so any solvent that isn't water is going to very strongly increase costs because the solvent is normally a very important part of the solutions by mass. Any other alternative is usually 10-100x more expensive than just pure water. Water is one of the few substances on this planet that you can get at mere cents per ton. Non-aqueous solvents can be eutectic solvents, ionic liquids or organic solvents.
-
@sepi Just that water is very cheap, so any solvent that isn't water is going to very strongly increase costs because the solvent is normally a very important part of the solutions by mass. Any other alternative is usually 10-100x more expensive than just pure water. Water is one of the few substances on this planet that you can get at mere cents per ton. Non-aqueous solvents can be eutectic solvents, ionic liquids or organic solvents.
@danielfp248 huh, that makes a lot of sense.
