Alternative Electrolytes
-
@danielfp248 said in Alternative Electrolytes:
To be honest, I am only interested in systems that use microporous membranes (no ion selective membranes) as selective membranes not only drive the initial cost of systems up a lot but can make them very unreliable, as even slight problems with selectivity can lead to solutions going to waste for non-symmetric electrolytes.
Me too, and that's the route I chose as well (trying to avoid chemistries that required them). Just wanted to ask in case you found an easy/inexpensive solution!
@danielfp248 said in Alternative Electrolytes:
Also note I've seen Fe oxides form at pH values as low as 2.5
Right, that's not too surprising either. Kinetics/rate of formation is difficult to deconvolute from thermodynamic "possibility to form" as it were. Just to clarify, seeing the oxides = orange particulates in the graphite felt?
Some other ideas:
- Does your anolyte yellow over time? I know we're ruling out crossover but that could be another easy way to check - FeCl3 is a deep yellow vs. FeCl2. I'm assuming you've thought of this but I thought I'd suggest it
- You mentioned seeing a lot of undissolved metal on the anode side. Any possibility of dead metal flaking off the felt? In acidic media I wouldn't expect it but who knows. What does the metal on the graphite felt look like usually? any pics?
Thanks for being so responsive. This is interesting
@muntasirms Thanks for your reply!
- Does your anolyte yellow over time? I know we're ruling out crossover but that could be another easy way to check - FeCl3 is a deep yellow vs. FeCl2. I'm assuming you've thought of this but I thought I'd suggest it
Chlorides here don't play a strong role as the Gly complexes are much stronger than the chloride ones. The Fe3+Gly complex is extremely red while the Fe2+Gly complex is transparent. There is always some additional Fe3+ - because my starting FeCl2 is slightly oxidized - so the starting electrolyte is already quite red. On charging the anolyte then turns transparent and the catholyte turns completely blood red. They never turn orange. On discharge the anolyte also never turns red, it remains transparent.
- You mentioned seeing a lot of undissolved metal on the anode side. Any possibility of dead metal flaking off the felt? In acidic media I wouldn't expect it but who knows. What does the metal on the graphite felt look like usually? any pics?
No, all the metal remains on the felt. I didn't get any peaks, but the metal looks just like regular plated Zn. It doesn't rust on exposure to oxygen, so it probably contains very little Fe. At Zn/Fe ratios higher than 1.5, Zn seems to plate exclusively, which I confirmed with CV measurements (I see no oxidation peaks for metallic Fe when doing CV, which you do see at lower ratios).
-
For a pointless waste of time search, I tried in google: "what is the best cheapest redox flow battery chemistry"
Came back with:High-energy and low-cost membrane-free chlorine flow battery:
https://www.nature.com/articles/s41467-022-28880-x#:~:text=To meet the needs of,La France in 188428.A high-energy and low-cost polysulfide/iodide redox flow battery:
https://www.sciencedirect.com/science/article/abs/pii/S2211285516304153#:~:text=Highlights * • The polysulfide/iodide redox flow,reversibility of polysulfide and iodide redox chemistries.Air-Breathing Aqueous Sulfur Flow Battery for Ultralow-Cost Long-Duration Electrical Storage:
https://www.sciencedirect.com/science/article/pii/S2542435117300326And 2 Google search options with a lot of results over my head:
Sulfur-Air Hybrid Redox Flow Batteries: https://www.google.com/search?num=10&cs=1&sca_esv=db6c98fd308e1745&q=Sulfur-Air+Hybrid+Redox+Flow+Batteries&sa=X&ved=2ahUKEwjS8bKDhoGPAxXpL0QIHXvhGwgQxccNegQIEhAB&mstk=AUtExfDy0ZazObNsltp9BYwtpQylX9u5AJT5yaQVQRbdARMJvJuEzcAf-Z2d4utwzTSnhCKcn4qOInT9b9zVkToTFJswogvnK4pun2X3x25KeDNW8dbrTS5jwMmIp6riqcFc6wG0Rm8FPMwC2B67TUxmok7FRMfFyfzTWq_Ub_BP2zY5rP4&csui=3Google sure like long URL's
-
@muntasirms Thanks for your reply!
- Does your anolyte yellow over time? I know we're ruling out crossover but that could be another easy way to check - FeCl3 is a deep yellow vs. FeCl2. I'm assuming you've thought of this but I thought I'd suggest it
Chlorides here don't play a strong role as the Gly complexes are much stronger than the chloride ones. The Fe3+Gly complex is extremely red while the Fe2+Gly complex is transparent. There is always some additional Fe3+ - because my starting FeCl2 is slightly oxidized - so the starting electrolyte is already quite red. On charging the anolyte then turns transparent and the catholyte turns completely blood red. They never turn orange. On discharge the anolyte also never turns red, it remains transparent.
- You mentioned seeing a lot of undissolved metal on the anode side. Any possibility of dead metal flaking off the felt? In acidic media I wouldn't expect it but who knows. What does the metal on the graphite felt look like usually? any pics?
No, all the metal remains on the felt. I didn't get any peaks, but the metal looks just like regular plated Zn. It doesn't rust on exposure to oxygen, so it probably contains very little Fe. At Zn/Fe ratios higher than 1.5, Zn seems to plate exclusively, which I confirmed with CV measurements (I see no oxidation peaks for metallic Fe when doing CV, which you do see at lower ratios).
@danielfp248 said in Alternative Electrolytes:
he Fe3+Gly complex is extremely red while the Fe2+Gly complex is transparent
Got it - I actually didn't know that about Fe-glycine complexes. Thank you for teaching me something new today. Could lend to some cool/inexpensive colorimetric characterization.
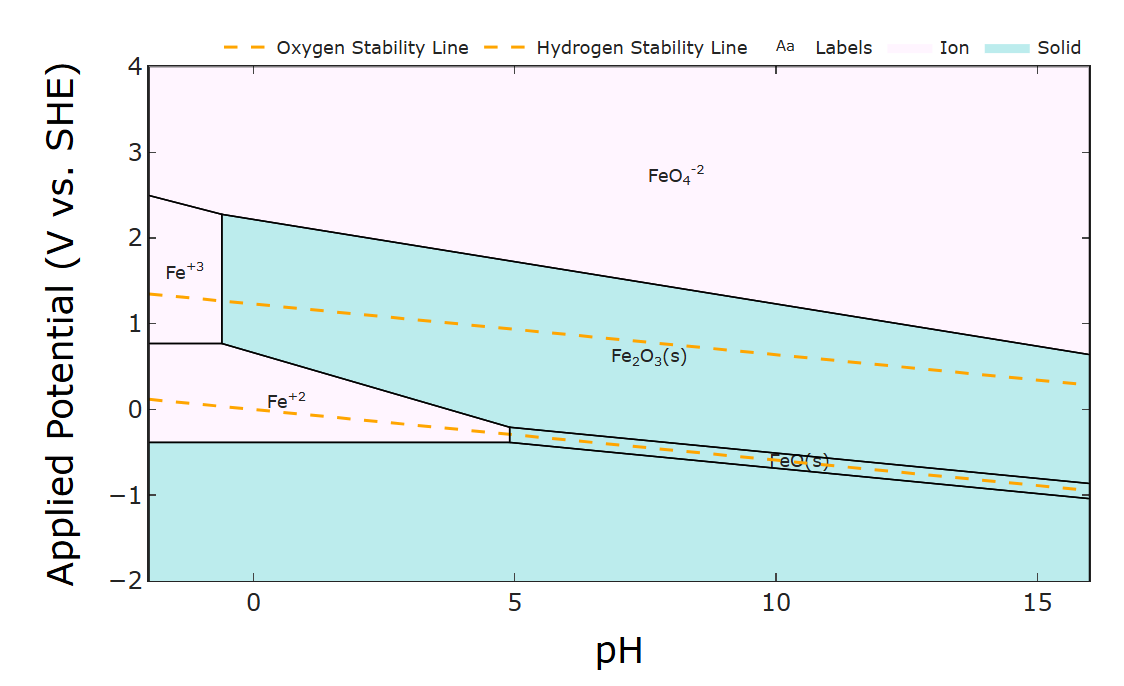
The plating makes sense given that Zn plating tends to be much faster than Fe plating. The main other thing I could think of is some invisible amount of oxide depositing over time and fouling the surface (may especially be difficult to see in the felt) since at high Fe2+ concentrations oxides will form (at least thermodynamically) according to its Pourbaix diagram (below). Of course kinetics of that deposition is all affected by complexing with glycine and the like so I'm not certain. Intuitively I would say it could be alleviated by decreasing the pH. I wish I could help more but thank you for being so responsive and detailed. Good luck!
-
For a pointless waste of time search, I tried in google: "what is the best cheapest redox flow battery chemistry"
Came back with:High-energy and low-cost membrane-free chlorine flow battery:
https://www.nature.com/articles/s41467-022-28880-x#:~:text=To meet the needs of,La France in 188428.A high-energy and low-cost polysulfide/iodide redox flow battery:
https://www.sciencedirect.com/science/article/abs/pii/S2211285516304153#:~:text=Highlights * • The polysulfide/iodide redox flow,reversibility of polysulfide and iodide redox chemistries.Air-Breathing Aqueous Sulfur Flow Battery for Ultralow-Cost Long-Duration Electrical Storage:
https://www.sciencedirect.com/science/article/pii/S2542435117300326And 2 Google search options with a lot of results over my head:
Sulfur-Air Hybrid Redox Flow Batteries: https://www.google.com/search?num=10&cs=1&sca_esv=db6c98fd308e1745&q=Sulfur-Air+Hybrid+Redox+Flow+Batteries&sa=X&ved=2ahUKEwjS8bKDhoGPAxXpL0QIHXvhGwgQxccNegQIEhAB&mstk=AUtExfDy0ZazObNsltp9BYwtpQylX9u5AJT5yaQVQRbdARMJvJuEzcAf-Z2d4utwzTSnhCKcn4qOInT9b9zVkToTFJswogvnK4pun2X3x25KeDNW8dbrTS5jwMmIp6riqcFc6wG0Rm8FPMwC2B67TUxmok7FRMfFyfzTWq_Ub_BP2zY5rP4&csui=3Google sure like long URL's
@Vorg One issue you will find is that many researchers will overhype their findings, so a lot of technologies might appear way better than they really are once you take a close look at them, especially when you think about using them at or above the kWh scale. All of the technologies you posted have very interesting characteristics - I've read probably all high impact flow battery papers from the last 20 years - but they all suffer from some strong handicaps that basically prevent their mass adoption. Because researchers at the base level do not usually deal with the problems of the kWh scale, they often neglect to account for these in meaningful ways. Even worse, some researchers realize these problems and write the papers in a way that obscures them (so that the paper "sells" the technology better). Sadly because of this, it requires high technical proficiency in the field to correctly evaluate these candidate technologies.
If you just googled you would believe that we have plenty of technologies that were much better than Vanadium, but reality shows only Vanadium flow batteries at large scale. This is part of why I believe our dev kit is important, to lower the entry bar for flow battery research enables people with other priorities to also research the technology and find and study solutions with the idea of scale up in mind, without the pressure to publish over them.
-
@danielfp248 said in Alternative Electrolytes:
he Fe3+Gly complex is extremely red while the Fe2+Gly complex is transparent
Got it - I actually didn't know that about Fe-glycine complexes. Thank you for teaching me something new today. Could lend to some cool/inexpensive colorimetric characterization.
The plating makes sense given that Zn plating tends to be much faster than Fe plating. The main other thing I could think of is some invisible amount of oxide depositing over time and fouling the surface (may especially be difficult to see in the felt) since at high Fe2+ concentrations oxides will form (at least thermodynamically) according to its Pourbaix diagram (below). Of course kinetics of that deposition is all affected by complexing with glycine and the like so I'm not certain. Intuitively I would say it could be alleviated by decreasing the pH. I wish I could help more but thank you for being so responsive and detailed. Good luck!

@muntasirms Thinking about the MgCl2/CaCl2 electrolytes, I started an experiment with 0.5M Fe, 0.5M Glycine and 5M ZnCl2. The thought is that ZnCl2 can also create water-in-salt electrolytes and furthermore, provide the reducing species for the anolyte in this case. I want to see if this degrades in the same way as the normal case with similar ratios of Fe/Zn. The high Zn will affect the Gly chelate formation though, but it should also make Fe hydroxide/oxide formation harder. I'll let you know how that goes. On preparation the solid is oily, orange and hazy, although it doesn't seem to contain any meaningful amount of precipitates.
-
Not surprising that dendrites start to become a problem with Zn concentrations this high. Even at a a capacity of 2 Ah/L I already see a dendrite piercing the microporous layer. Because of the fact that we have reactions of catholyte with the Zn these dendrites are "self-healing" to a degree, but of course charge is lost as these dendrites get consumed.
I am trying to charge to 5Ah/L which would be 75% SOC of a 0.5M symmetric electrolyte.
-
I stopped the charging process and extracted the charged anolyte and catholyte (anolyte left, catholyte right), which you can see below. The catholyte is definitely not clear, but the amount of solid seems small. On addition of 1mL of HCl and mixing the solution becomes clear and yellow (as expected). I will now try cycling this, although I'm afraid it's too acidic for Zn to be stable (although who knows at Zn concentration this high).

-
Once you get it too acidic it really doesn't work anymore. No metal plates at all as the Zn just reacts with the acid to generate H2. Ohmic resistance also increases a lot with cycling.
-
I am now testing 7g ZnCl2, 0.84g FeCl2.2H2O and 0.8g Glycine plus 7mL of water, which gives around 10mL total volume (measured with a syringe). This is around 6M Zn, 0.5M Fe, 1M Glycine. This is how the electrolyte looks on preparation:

I am now testing it with a non-conductive felt on the Zn side, to increase the time it takes for dendrites to form, as they now have to cross the entire cell to reach the membrane. I expect some loss in conductivity but this can be worth the tradeoff. This also increases the time it takes for Fe3+ to react with Zn as it now has to entirely cross the separator and cannot easily meet Zn half way through.
As you can see the solution is not entirely translucid, because there is some slight Fe oxide impurity in the FeCl2 I use.
-
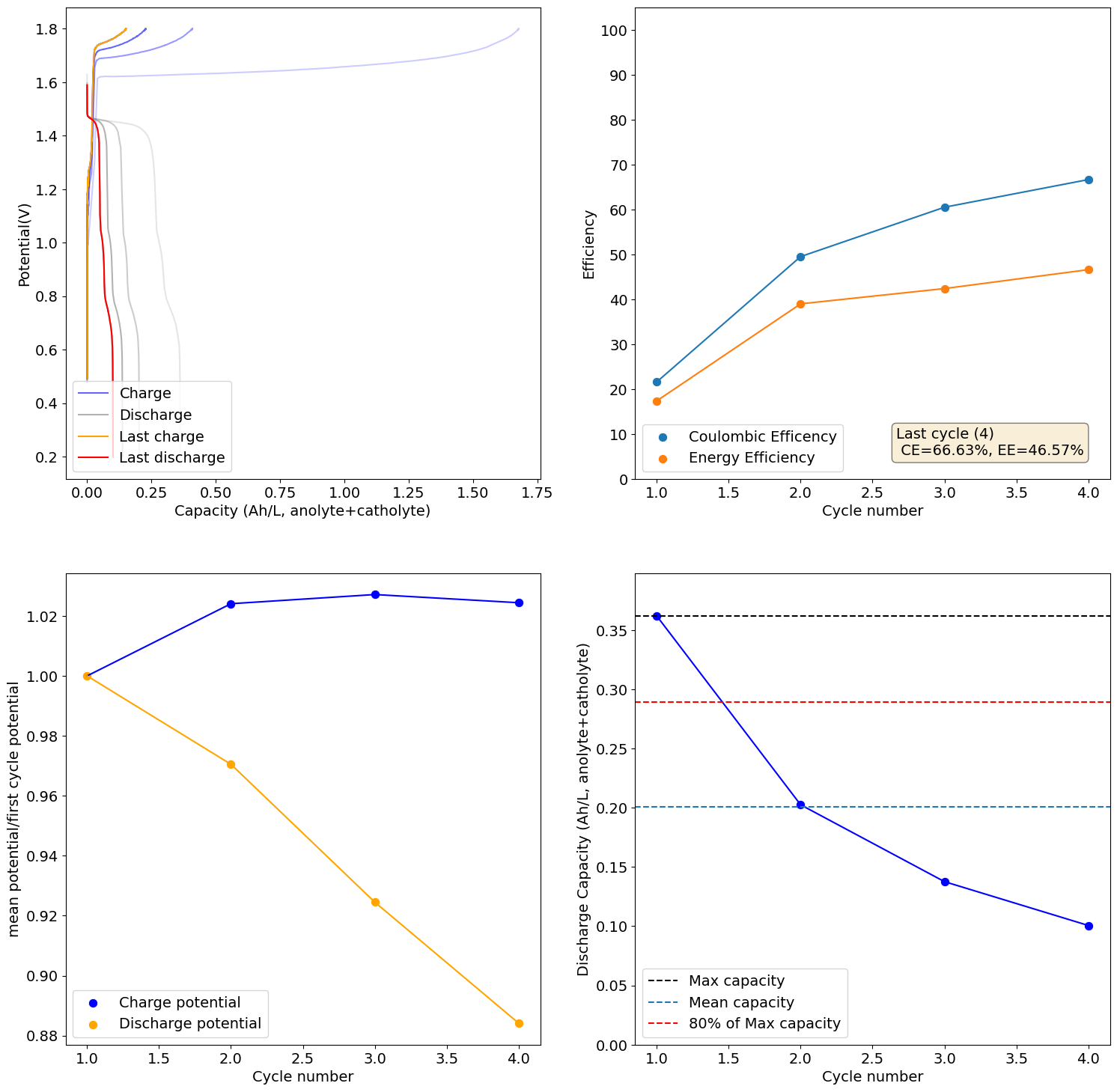
Charging to 2Ah/L (~30% of SOC at 0.5M Fe) shows promising results, although CE and EE are still quite low. I will try charging to 5Ah/L next.
-
I also prepared a test electrolyte with 6.8g of ZnCl2, 3.2g of FeCl2.2H2O, 2.25g of Glycine with 5.5mL of water to reach a volume of 10mL and concentrations of 5M Zn, 2M Fe and 3M Glycine. The electrolyte is deep red as shown below. The 100% SOC mark for this electrolyte would be ~26Ah/L but I would honestly be more than happy if it cycled to 15Ah/L in a stable manner. I will test this electrolyte once I'm done testing the 0.5M Fe electrolyte I'm running atm.

-
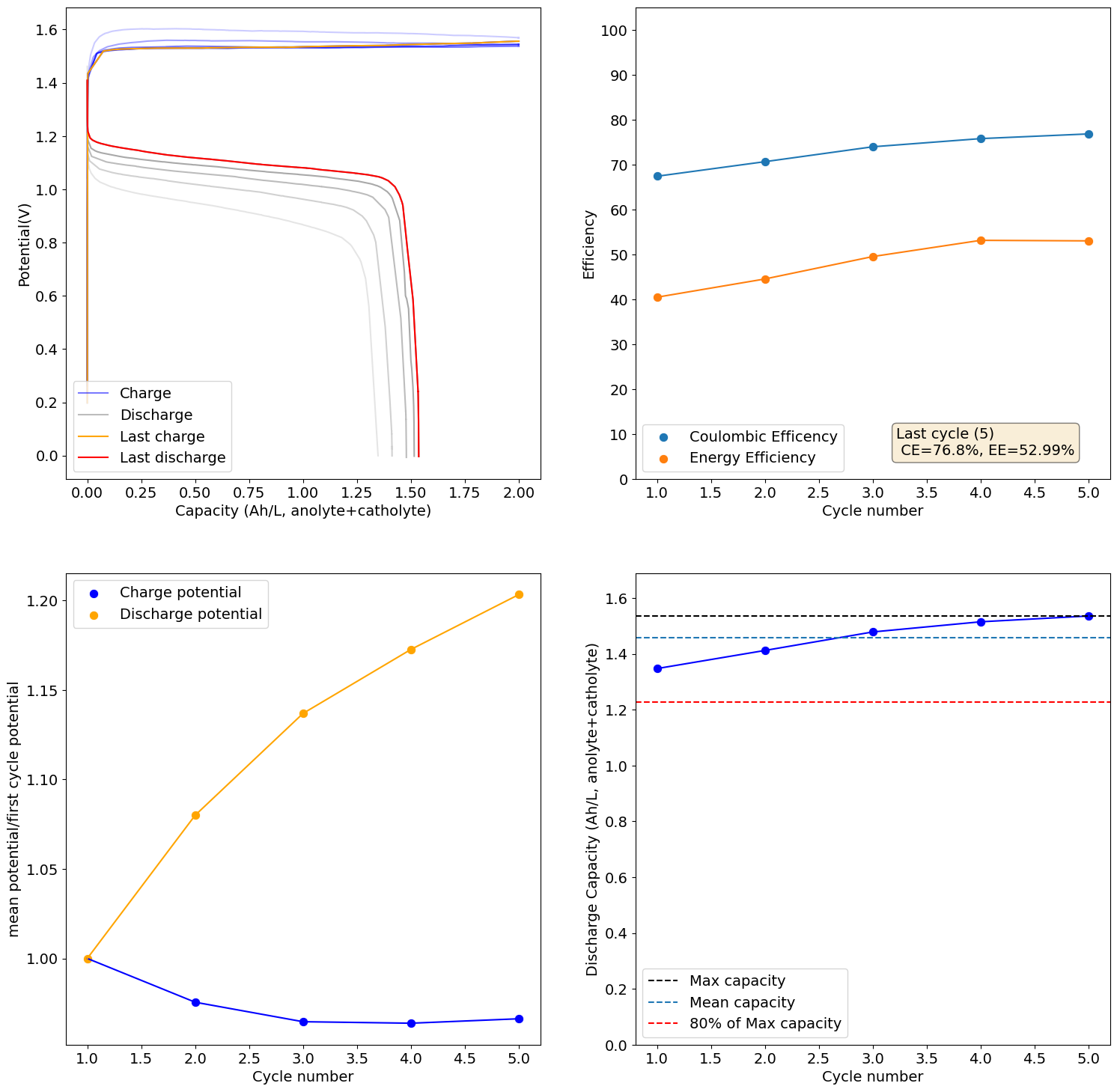
The 0.5M Fe solution at a current density of 5mA/cm2 reached the Nernst limit at around 3.1Ah/L. This is the first cycle:
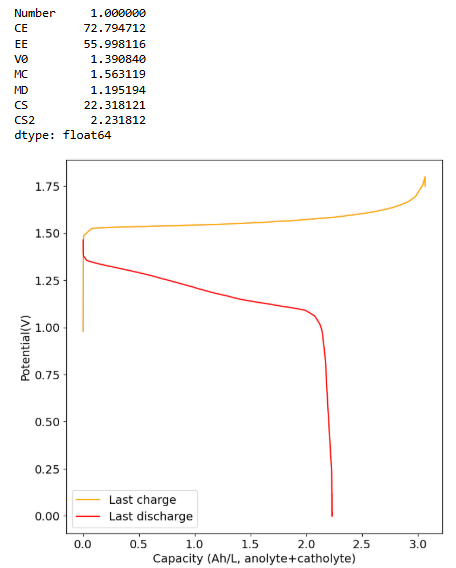
I will keep you posted on how the cycling goes and if it degrades in capacity as other similar tests have done in the past.
-
Same result as previous times, significant decreases in capacity as a function of cycling:
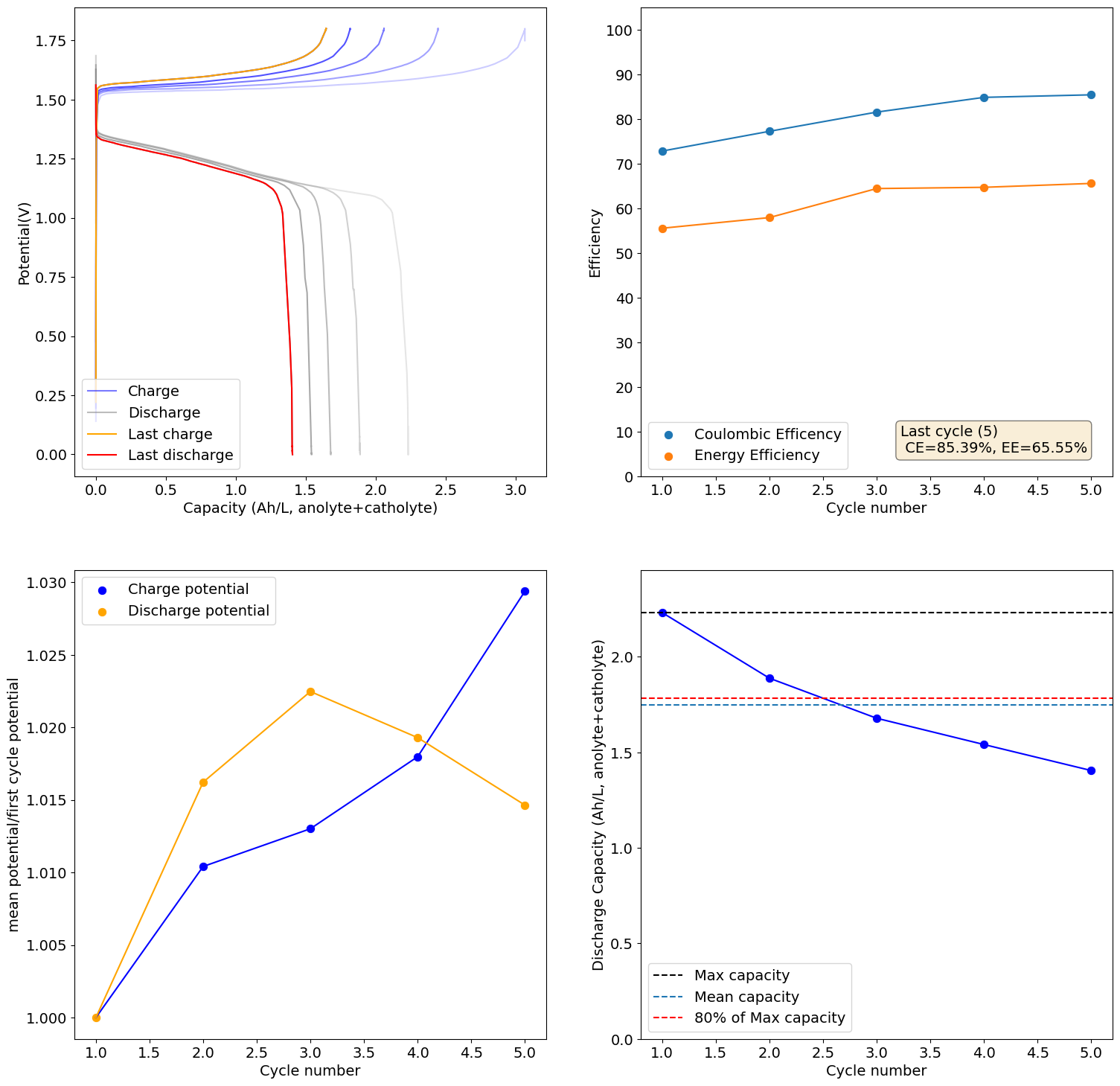
-
Question, what about using some sort aluminum oxide? Tesla is rumored to be using a new aluminum battery the developed in place of LFOP for the new model 2. They said something about aluminum holding 3 ions? per molecule instead of 1. I poked around and there are groups working on aluminum flow batteries. Seem they don't have the dendrite problem.
I see a number of groups working on aluminum flow batteries. Seems they don't have the dendrite problem.
-
Question, what about using some sort aluminum oxide? Tesla is rumored to be using a new aluminum battery the developed in place of LFOP for the new model 2. They said something about aluminum holding 3 ions? per molecule instead of 1. I poked around and there are groups working on aluminum flow batteries. Seem they don't have the dendrite problem.
I see a number of groups working on aluminum flow batteries. Seems they don't have the dendrite problem.
@Vorg Aluminum would be great in that it is a trivalent cation, so you get 3 electrons per Al atom, one of the most efficient atoms in this manner. A rechargeable Al/air battery has one of the highest theoretical energy densities possible, especially if the oxygen would come from the air. However aluminum is incredibly hard to reduce, so it is one of the trickiest batteries to get into a rechargeable form. In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems. I don't believe this is something we could realistically achieve, given the complexities of this chemistry. In my opinion there are lower hanging fruit, but of course, anyone who wants to try it and share is welcome!
-
Maybe that is what the video was talking about when they said the biggest problem is ion lock, or maybe it was electron lock. But basicly it's very hard to get the charge out at a useful rate. I'm sure there is more to Tesla's secret sauce, but they said Tesla's fix was very thin aluminum with carbon pressed into it creating a battery that gave it's charge better, could be charged in 5 minutes, and produced far less heat then lithium batteries with no thermal runaway problem. It sounded like they won't even use battery cooling for it because what heat it does produce makes it work better. A lot of "sounds good", but will see when it hits the road.
-
@Vorg Aluminum would be great in that it is a trivalent cation, so you get 3 electrons per Al atom, one of the most efficient atoms in this manner. A rechargeable Al/air battery has one of the highest theoretical energy densities possible, especially if the oxygen would come from the air. However aluminum is incredibly hard to reduce, so it is one of the trickiest batteries to get into a rechargeable form. In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems. I don't believe this is something we could realistically achieve, given the complexities of this chemistry. In my opinion there are lower hanging fruit, but of course, anyone who wants to try it and share is welcome!
@danielfp248 said in Alternative Electrolytes:
In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems.
What's expensive about the non-aqueous media? Do you speak about deep eutectic solvents?
-
Maybe that is what the video was talking about when they said the biggest problem is ion lock, or maybe it was electron lock. But basicly it's very hard to get the charge out at a useful rate. I'm sure there is more to Tesla's secret sauce, but they said Tesla's fix was very thin aluminum with carbon pressed into it creating a battery that gave it's charge better, could be charged in 5 minutes, and produced far less heat then lithium batteries with no thermal runaway problem. It sounded like they won't even use battery cooling for it because what heat it does produce makes it work better. A lot of "sounds good", but will see when it hits the road.
@Vorg This is a different type of battery technology though, it is aluminum Ion, using thin film materials. This type of battery chemistry isn't really compatible with a flow battery mechanic.
-
@danielfp248 said in Alternative Electrolytes:
In aqueous media nobody has really achieved it at energy densities that would matter and in non-aqueous media it is incredibly expensive and still, not without reversibility problems.
What's expensive about the non-aqueous media? Do you speak about deep eutectic solvents?
@sepi Just that water is very cheap, so any solvent that isn't water is going to very strongly increase costs because the solvent is normally a very important part of the solutions by mass. Any other alternative is usually 10-100x more expensive than just pure water. Water is one of the few substances on this planet that you can get at mere cents per ton. Non-aqueous solvents can be eutectic solvents, ionic liquids or organic solvents.
-
@sepi Just that water is very cheap, so any solvent that isn't water is going to very strongly increase costs because the solvent is normally a very important part of the solutions by mass. Any other alternative is usually 10-100x more expensive than just pure water. Water is one of the few substances on this planet that you can get at mere cents per ton. Non-aqueous solvents can be eutectic solvents, ionic liquids or organic solvents.
@danielfp248 huh, that makes a lot of sense.
