@danielfp248 said in Alternative Electrolytes:
I didn't see any Fe oxides forming
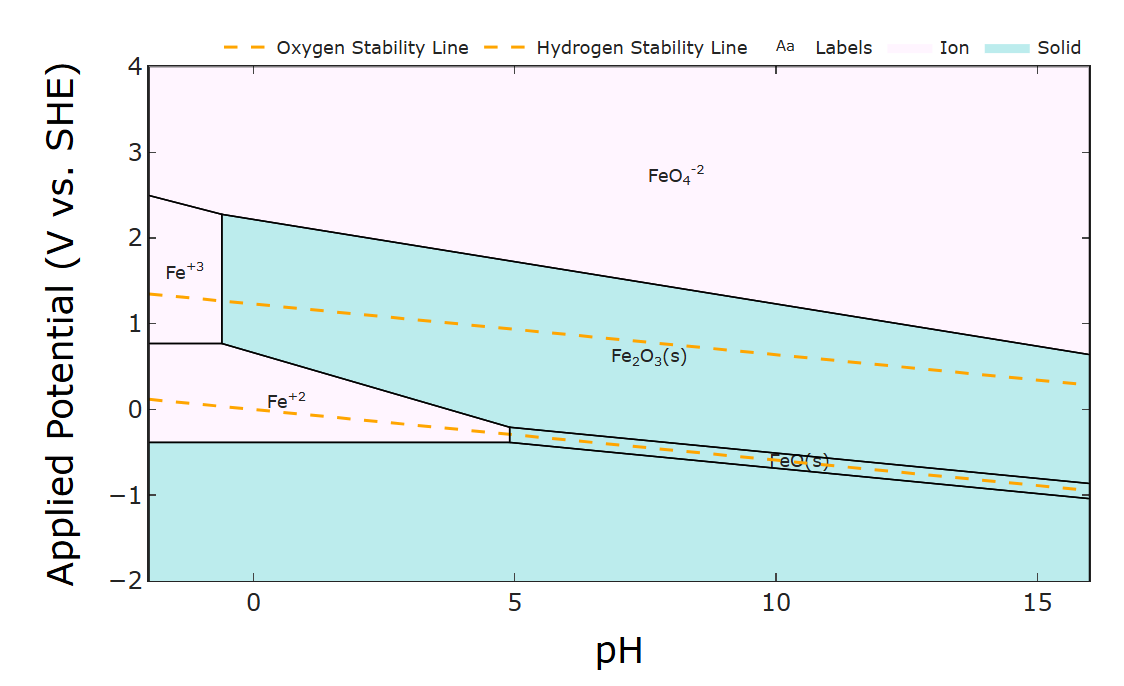
This aligns with what I'd expect - most iron oxides form at pHs above ~ 5 ish. The most stable ones (magnetite is usually the major problem) form in strongly basic conditions, ph >12 ish.
@danielfp248 said in Alternative Electrolytes:
This is 2M FeCl2, 3M ZnCl2, 2M Glycine. At 20mA/cm2. Felt on both sides, daramic membrane. The pH of this is 3.2.
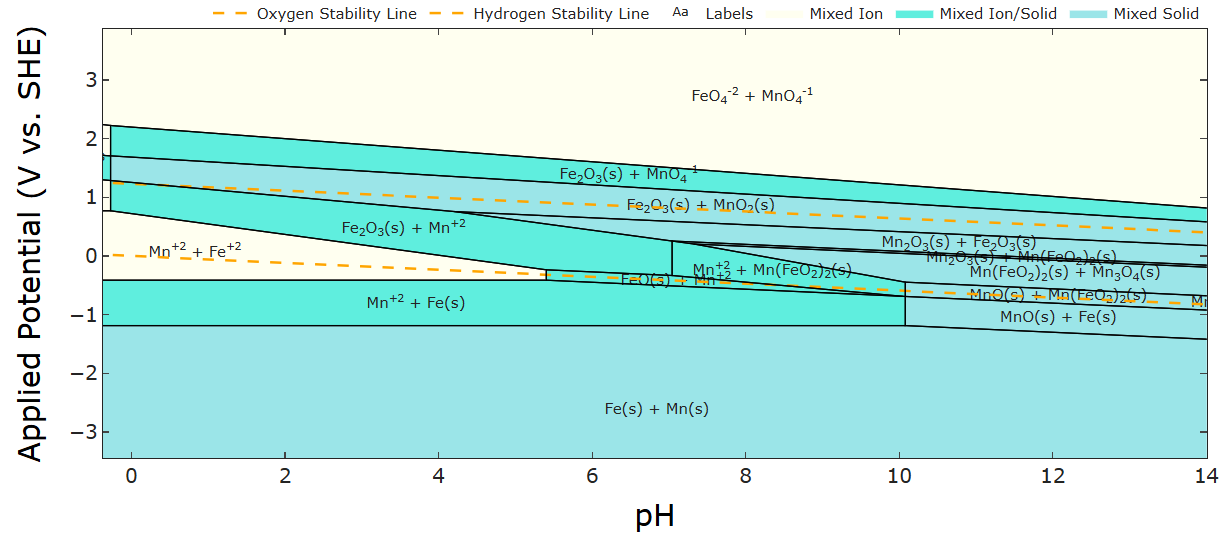
Hmm...your daramic membrane isn't ion-selective, right? Is it just a size exclusion separator? My first thought is ion crossover (or if you already have both electrolytes mixed, just corrosion). Similar to my question in the Fe-Mn post actually! If your separator isn't ion selective (or you already have all active species mixed together), then any Fe2+ and Fe3+ in electrical contact with Zn metal can drive galvanic corrosion because their reaction voltages differ:
2Fe3+ + Zn <-> 2Fe2+ + Zn2+
A couple ways you could ad-hoc test this:
- After charging, let the cell rest but track the open circuit potential over time - if the cell voltage drops fairly quickly (over the course of hours), that could suggest corrosion
- If you want to be more quantitative, you could take a look at mixed potential theory - it predicts what the open circuit potential of a system would be if you have two redox couples that are electrically shorted. Here's a nice preprint from a buddy at @quinnale 's lab that explains it nicely (though fair warning, it's still complicated).
- Try having two separate solutions of the FeCl2+Glycine and ZnCl2+Glycine in each half cell. Unless you have an ion selective membrane, you'll still have ion crossover over time, but it will be slower and your capacity loss shouldn't be as significant.
I think this also explains your lower, but very stable coulombic efficiency.
Granted, if that hypothesis is right, you would've seen this problem from the first cycle. So someone else might have a more accurate view of the problem! Do you guys have the equipment for 3 electrode experiments? That could help with diagnostics. I might make a new post with a list of affordable electrochemical equipment like reference electrodes that I've run into.