Thanks for sharing your pictures! It always makes us very happy to see anyone reproducing the kit independently.
You can see my current setup below:
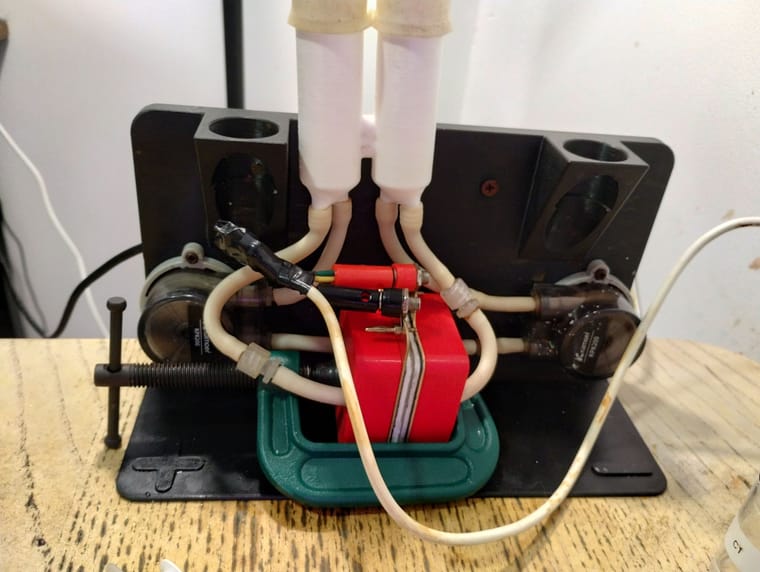
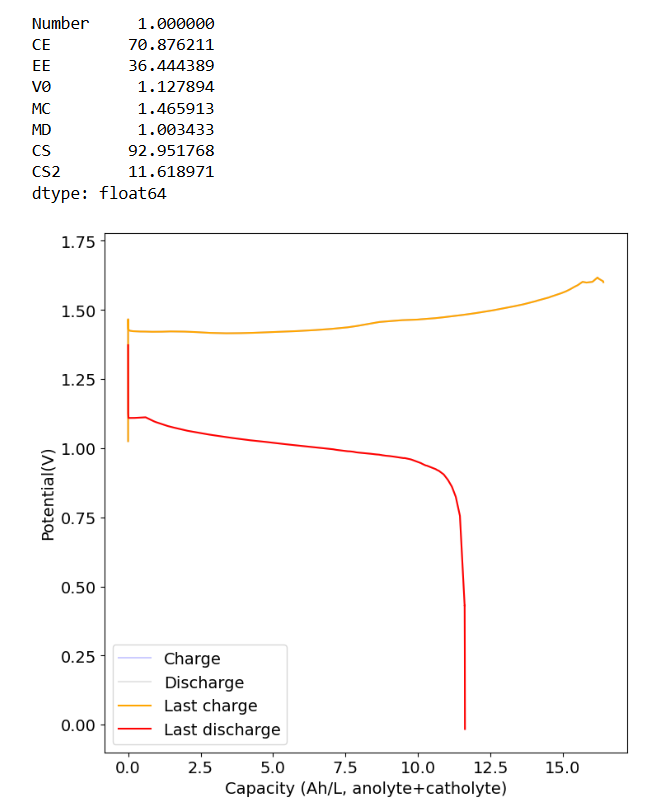
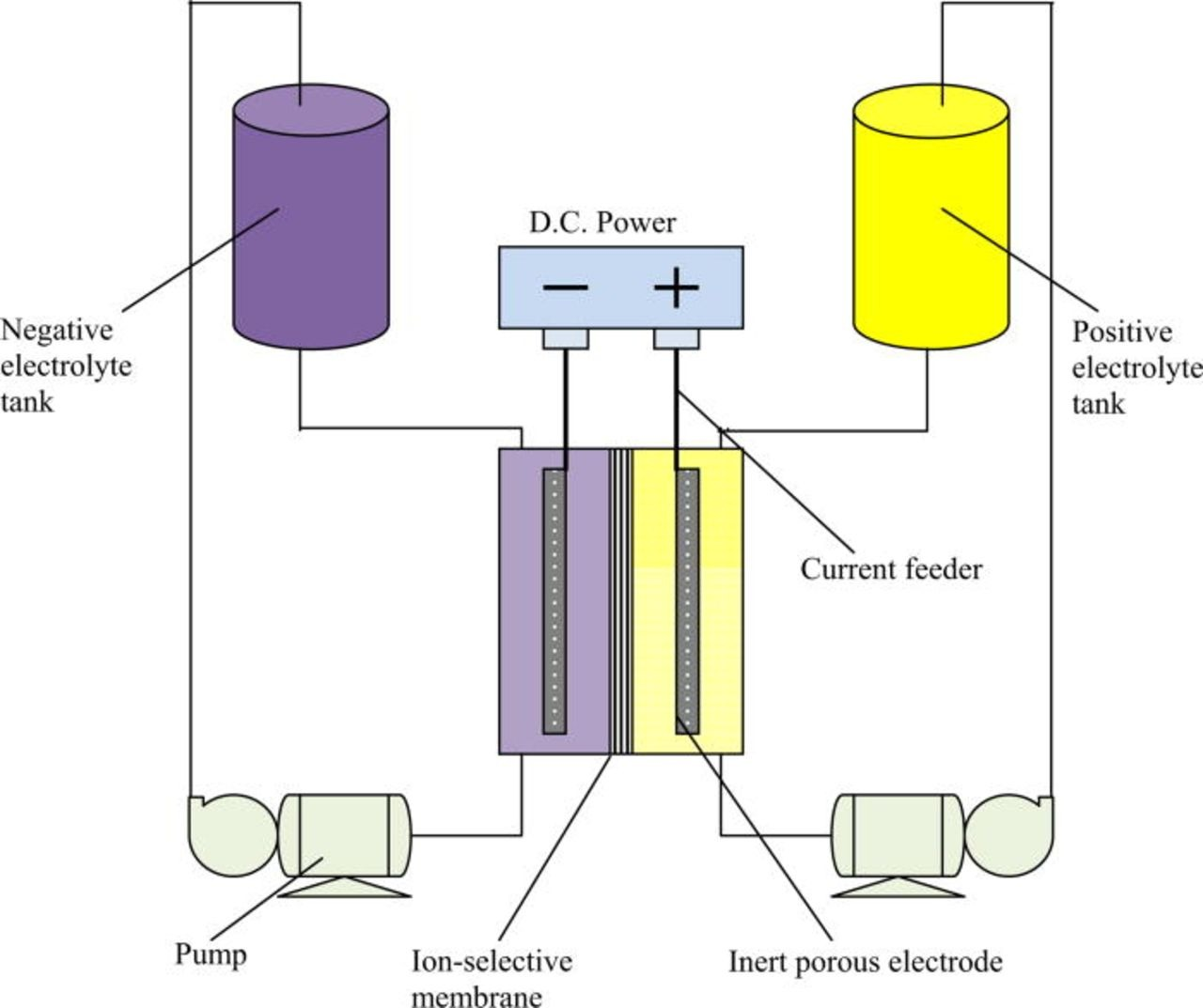
I am testing the double reservoir with spill-over communication (that's why the reservoirs look different and are at the center). My pumps are connected in a suction configuration and they enter and exit the cell on their sides. When you have pumps in a push-through configuration having the flow going from bottom to top is important to push air out, but when you suck through the cell the vacuum will push all air out and fill the entire cavity, almost regardless of how you pump. I haven't seen any air being trapped there (no bubbles are evident when shaking or moving the cell so that the flow is in either direction).
As you can see I have changed some connections to luer locks, except the connections for the pumps. I however like how you've made them ALL luer locks, much simpler to disassemble.
As you can see I'm also testing the new clamp-compatible end-plates and clamp. This works SO much easier than the screws, since the compression is centered I am also experiencing fewer problems with the cell and slightly better energy efficiencies since the felt compression seems to be more uniform. Opening and closing this cell is a breeze compared to the screws. I used PLA with 80% infill to print the end plates but I am sure PETG will work great too.
About the PP, it is a bit tricky to find the PP and printer settings that work best for water tight results. I am using the Ivor white PP from smart 3d, which has worked well on the prusa core one. In any case, let me know if there's anything I can help you with.